Bunsen burners have been a fundamental tool in laboratories for centuries, allowing scientists to perform various experiments and analyses. The mesmerizing blue flame they produce holds intricate chemistry within its flickering dance. The invention of the Bunsen burner is a significant milestone in the field of chemistry. This revolutionary device was developed by German chemist Robert Wilhelm Bunsen in the 19th century. In this exploration, we’ll uncover the scientific principles that govern Bunsen burner flames and delve into the chemistry behind their colors and characteristics.
Combustion: The Core Process
At the heart of every Bunsen burner flame lies the process of combustion. Combustion occurs when a substance reacts with oxygen gas (O2) in the air, producing heat, light, and new chemical compounds. In the case of a Bunsen burner, the fuel source is typically natural gas or liquefied petroleum gas (LPG), which reacts with oxygen to generate the flame.
The Flame Structure
A Bunsen burner flame consists of several distinct zones:
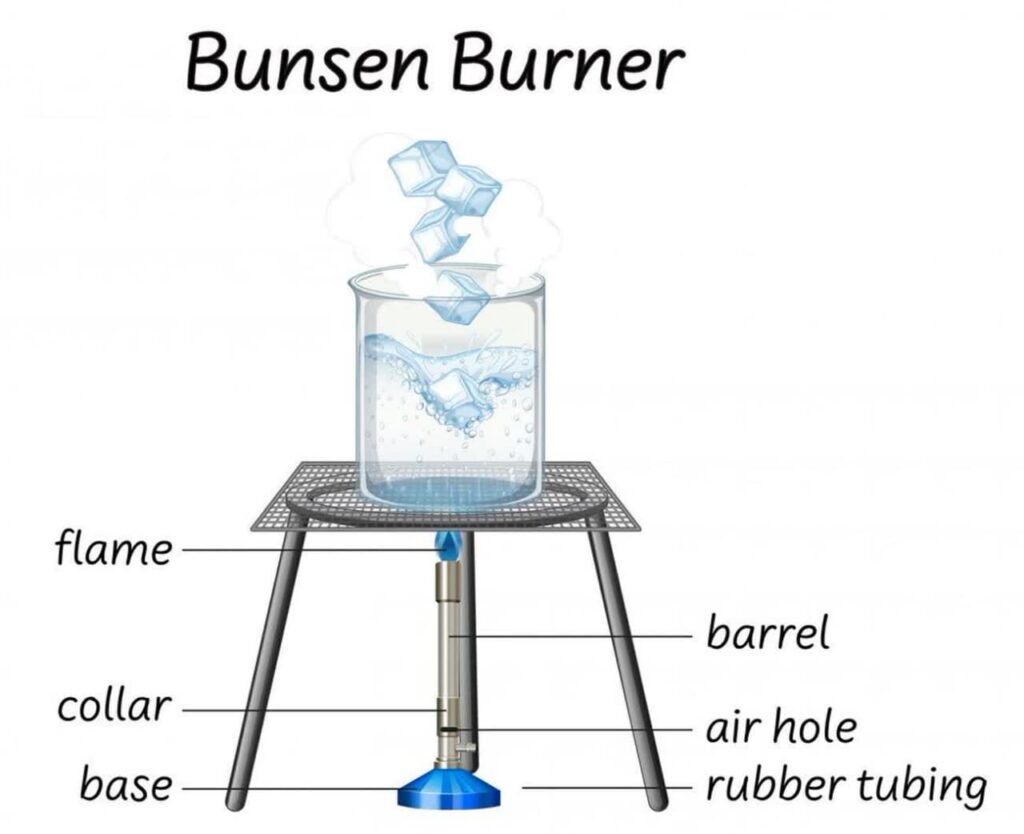
Outer Cone: The Blue Zone
The outer cone of the Bunsen burner flame is blue and represents complete combustion. Here, the gas molecules are fully oxidized, forming water vapor (H2O) and carbon dioxide (CO2). The blue color results from excited and energized gas molecules emitting light in the blue part of the spectrum as they return to their normal state from higher energy levels.
Inner Cone: The Darker Blue Zone
The inner cone of the flame appears darker blue due to a higher concentration of fuel molecules. In this zone, combustion is not as complete, leading to the presence of carbon particles. The darkness of this zone indicates the presence of unburned or partially burned fuel.
Outer Envelope: The Lighter Blue Zone
Surrounding the inner cone is the outer envelope, which is a lighter blue color. Here, additional air mixes with the unburned fuel, allowing for more complete combustion. However, this zone still contains some unburned particles, leading to a less intense blue hue.
Luminous Zone: The Innermost, Yellow Zone
The luminous zone, closest to the base of the flame, appears yellow due to incandescence. In this zone, the combustion is incomplete, and tiny carbon particles are heated to a glowing state, emitting a yellow-orange light. This zone is often referred to as the “safety flame” because it is less hot and produces minimal heat.
Adjusting the Flame of Bunsen Burner
Laboratory technicians can adjust the Bunsen burner flame by controlling the air and gas supply:
– Air Regulation: By adjusting the air intake, the technician can control the ratio of oxygen to fuel. More air results in complete combustion, producing a hotter, blue flame. Less air creates a fuel-rich flame with a yellow hue.
– Gas Supply: Controlling the gas flow determines the flame’s height and intensity. Higher gas flow produces a larger, more intense flame, while reducing the gas flow creates a smaller, gentler flame.
Practical Applications
The Bunsen burner is a versatile laboratory tool that finds applications in various scientific experiments and processes. One of its primary uses is in heating substances during chemical reactions. The adjustable flame of the Bunsen burner allows scientists to control the temperature precisely, ensuring optimal conditions for reactions to occur. This is particularly important in experiments that require specific temperature ranges for successful outcomes.
Another application of the Bunsen burner is in sterilization processes. In microbiology and medical laboratories, the burner is used to sterilize equipment such as inoculating loops and test tubes. By passing these items through the flame, any potential contaminants are eliminated, ensuring a sterile environment for experiments and procedures.
Furthermore, the Bunsen burner is also utilized in flame tests. This technique is employed to identify the presence of certain elements in a compound. By introducing a small amount of the compound into the flame, the burner produces characteristic colors that are unique to specific elements. This allows scientists to identify the composition of unknown substances, aiding in chemical analysis and research.
Understanding the chemistry behind Bunsen burner flames is crucial in various scientific applications. For instance, in analytical chemistry, the type of flame used can affect the outcome of experiments. A reducing flame (fuel-rich) is employed for certain chemical tests, while an oxidizing flame (oxygen-rich) is necessary for others.
In summary, the Bunsen burner is an indispensable tool in scientific laboratories. Its applications range from heating substances during chemical reactions to sterilizing equipment and conducting flame tests for element identification. Its versatility and precise control over temperature make it an essential instrument for various scientific experiments and processes.
Conclusion
The Bunsen burner flame, with its vibrant colors and intricate structure, serves as a fascinating example of the chemical processes occurring in our everyday scientific endeavors. Mastering the art of adjusting the flame allows scientists to tailor their experiments, showcasing the fusion of chemistry and practical application in the laboratory setting.